According to Bohr's Model of the Atom Electrons Behave Like
Electrons behave like waves and particles this is characterised by the wave side in the Bohr model and can be diffracted yet it will collide with other particles. When the number of protons and electrons possessed by an atom are unequal the atom _____.

Atom Atomic Mass And Isotopes Britannica
Neils bohr said electrons revolve around the nucleus in a particular path called as Orbit which are elliptical in shape.
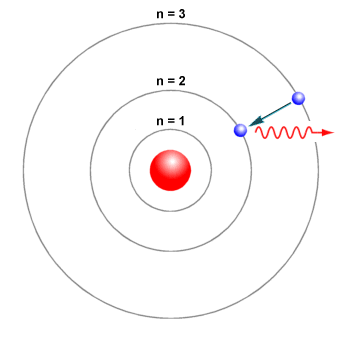
. Bohr Model 7 points According to the Bohr atomic model point-like electrons orbit a point-like nucleus at particular fixed radii. Postulates of Bohrs Model of an Atom. Each orbit or shell has fixed energy and is called an orbital shell.
In one fixed orbit at all times. Unlike the modern model of the atom Bohrs model states that a. The model proposes that the maximum number of electrons that can be accommodated in any particular orbit is 2n 2 where n is the number of orbits.
In specific allowed orbits. Initially an electron is in orbital 1 at a radial distance R 59 x 10-10 m from the. According to the modern model of the atom a.
Is shown in the figure below. Electrons behave like waves. When the electron is in one of these orbits its energy is fixed.
Energy levels are represented by an integer n1 2 3. Moving electrons form an electron cloud b. Bohrs Model is an atomic model proposed by a Danish Physicist Niels Bohr in 1913.
In an atom electrons that are negatively charged revolve around the positively charged nucleus in a circular path called orbits or shells. In bohrs model of the atom where are the electrons and protons located. Suatu atom dengan nomor atom 53 dan massa atom 127 mengandung.
According to the Bohr model of the atom the single electron of a hydrogen atom circles the nucleus. Boggs model of the atom electrons behave like atomic theory stated that every element was made of atoms that could not be subdivided atoms of. Where as rutherfords Model of an atom tells us the electrons are present randomly in an atom like how seeds present in watermelon.
At any of an infinite number of distances depending on its energy. Here seeds are considered electrons and pulp as protons and neutrons. The electron cloud model describes the _____ of electrons in an atom.
Electrons move in set paths around the nucleus of an atom. Atoms cannot be divided into smaller parts. Electrons and protons circle neutrons c.
According to this model In an atom the electrons revolve around the nucleus in definite energy levels called orbitsshells. According to the Bohr model often referred to as a planetary model the electrons encircle the nucleus of the atom in specific allowable paths called orbits. An example of such an atom with two electron orbitals and a nucleus of charge Q 1536 x 10-17C.

Atomic Structure Protons Electrons Neutrons Youtube

Atom Orbits And Energy Levels Britannica

Lesson Explainer Modern Atomic Theory Nagwa

Can You Strip Away Electrons Completely From An Atom Quora
Discovering The Atom A Brief History

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

Electrons In Atoms Bohr Orbits Vs Electron Cloud Orbitals Ppt Download

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

The Quantum Mechanical Model Of The Atom Article Khan Academy

Electron Cloud Atomic Model Read Chemistry Ck 12 Foundation

Atom Orbits And Energy Levels Britannica

Electrons Biology For Majors I

The Structure Of The Atom Astronomy

Warm Up 2 10 What Do You Know About Atoms And The Periodic Table What Do You Think An Atom Looks Like Ppt Download




Comments
Post a Comment